First place! YanengBIO topped the national EQA results once again
![]() 0
0
![]() 2022-08-26
2022-08-26
Undoubtedly, YanengBIO is still in the FIRST PLACE in 2022 EQA of HPV genotyping tests. Results were released by China National Center for Clinical Laboratories.
Of the 1,642 laboratories participating, 371 used our products, ranked the first again. Thereinto, 360 laboratories passed the EQA with full marks. And the results of 10 laboratories are between 99-80. The overall pass rate is up to 100%.
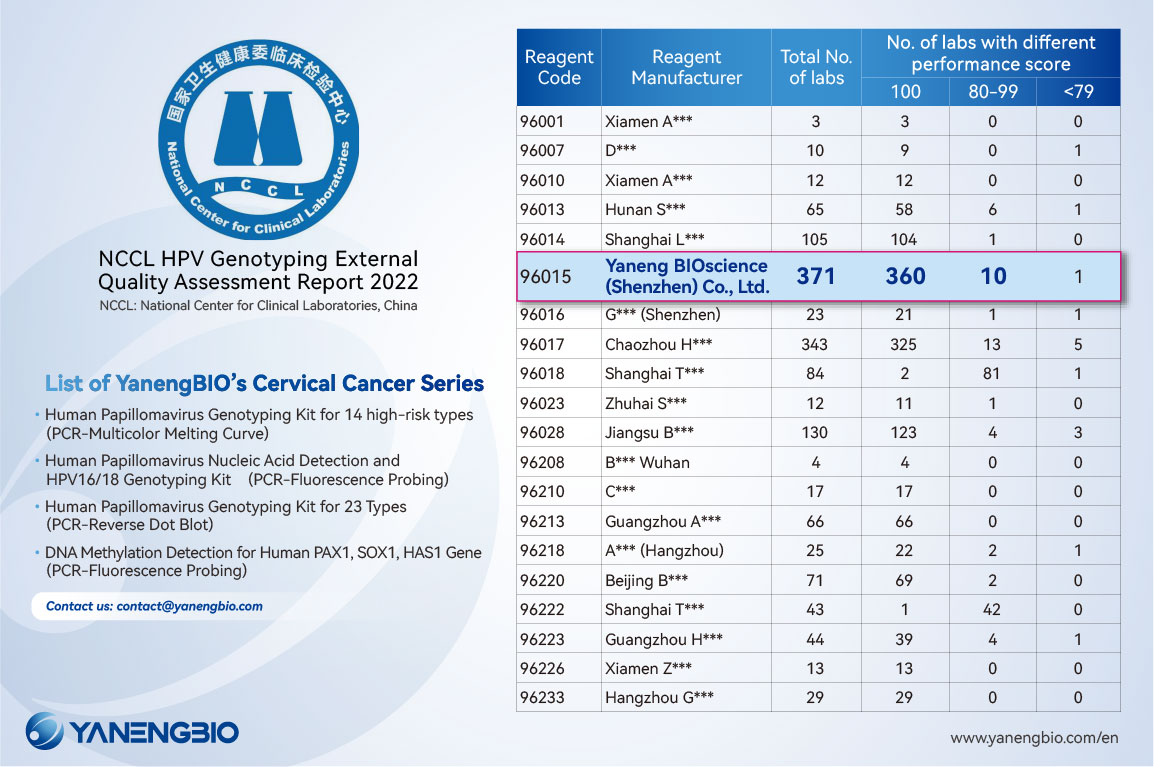
External quality assessment (EQA), also known as proficiency verification experiments, is an important part of the total quality management of recognized clinical laboratories and an important basis for laboratory test quality accreditation. The application department for EQA is divided into the Provincial Clinical Laboratory Center and the National Health Commission Clinical Laboratory Center, which is currently the highest standard for laboratory capacity evaluation in China.
As a leading enterprise in the field of HPV testing in China, YanengBIO will continue to be committed to the cause of women's health in the world. In the future, we believe that we will certainly be able to provide more help to women in both HPV genotyping testing and cervical cancer prevention.
List of YanengBIO’s Cervical Cancer Series
· Human Papillomavirus Genotyping Kit for 14 high-risk types (PCR-Multicolor Melting Curve)
· Human Papillomavirus Nucleic Acid Detection and HPV16/18 Genotyping Kit (PCR-Fluorescence Probing)
· Human Papillomavirus Genotyping Kit for 23 Types (PCR-Reverse Dot Blot)
· DNA Methylation Detection for Human PAX1, SOX1, HAS1 Gene (PCR-Fluorescence Probing)